Patient-Based Therapeutics
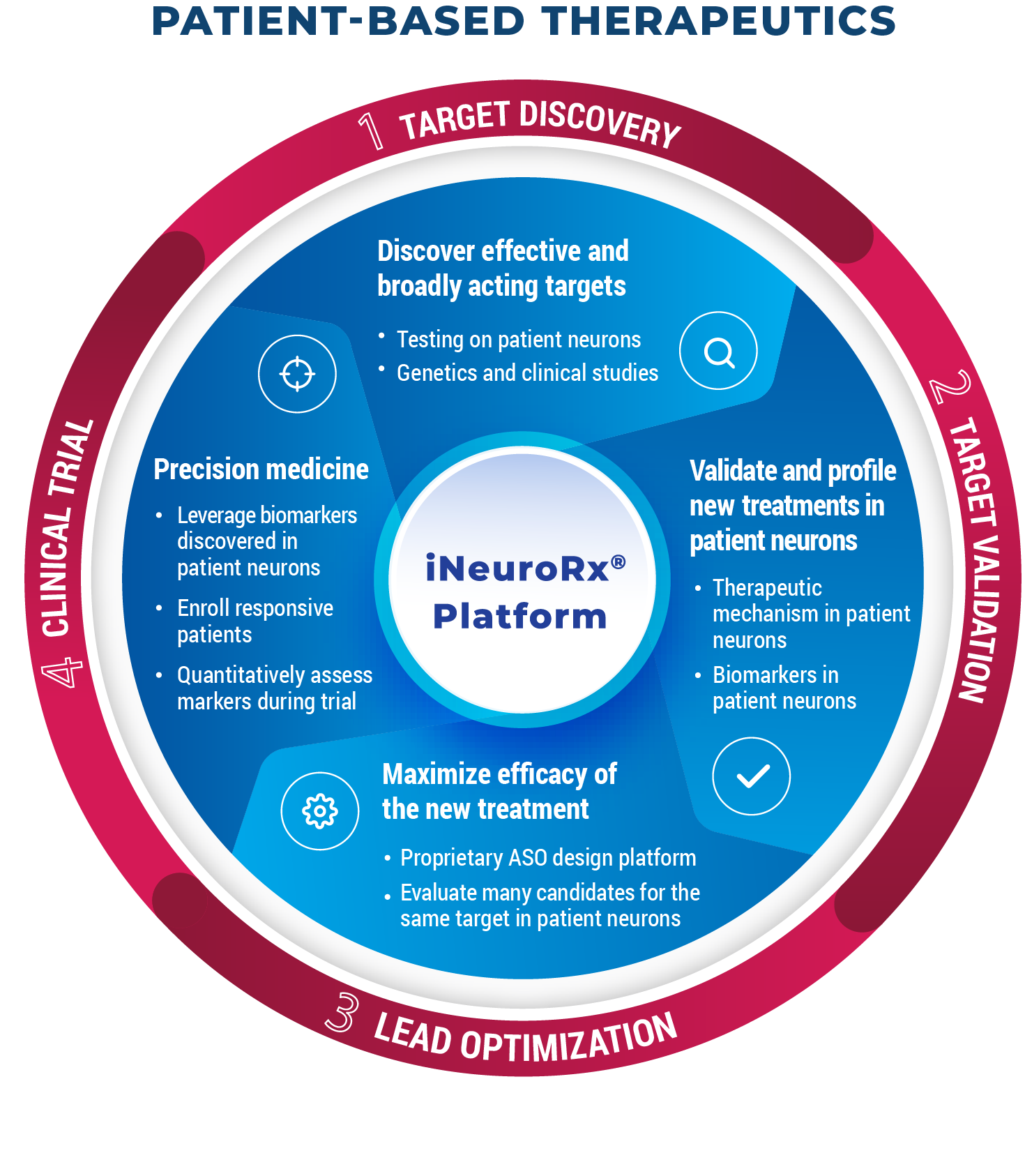
Many scientists believed a single, effective treatment for all patients was impossible, and instead precise treatments targeting multiple, rare, often genetically defined forms of disease would be required; but AcuraStem is showing how this can be done using its iNeuroRx® technology platform. This approach leads to treatments that are more likely to be effective and work for all patients, not limited to rare subtypes (e.g. C9ORF72-ALS / FTD).
Patient-Based Therapeutics

There are no good animal models of neurodegenerative diseases such as ALS and FTD. For example, mouse models of TDP-43 pathology (which occurs in ~ 97% of ALS patients and ~ 45% of FTD patients) fail to accurately recapitulate pathology arising from both gain- and loss-of-function of TDP-43.
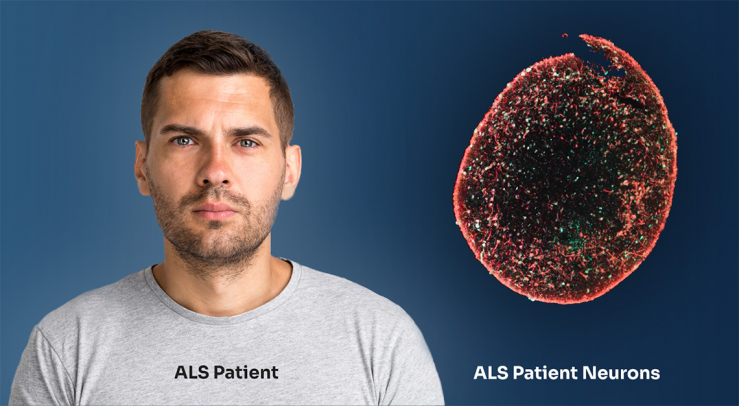
AcuraStem is an inventor and leader in generating models of individual patients that accurately reflect disease (ALS, FTD and CMT). The model consists of living neurons and supporting cells such as glia and astrocytes in a dish that we can study. The neurons are created from patient samples, such as blood or skin punch biopsies, using AcuraStem’s cellular reprogramming technologies.
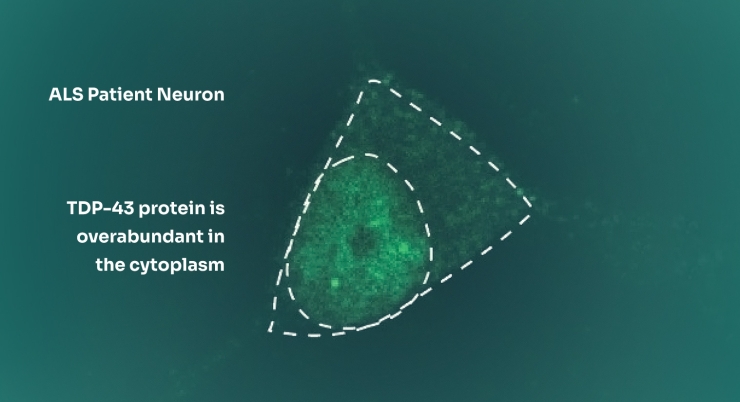
These patient-specific models accurately reflect key aspects of disease, such RNA processing errors (e.g. in STMN2 or UNC13A), TDP-43 mislocalization from the nucleus to the cytoplasm and neurodegeneration. The problems arise naturally from the patient’s DNA. This is a very different approach from animal models where the pathology is engineered into the mouse.
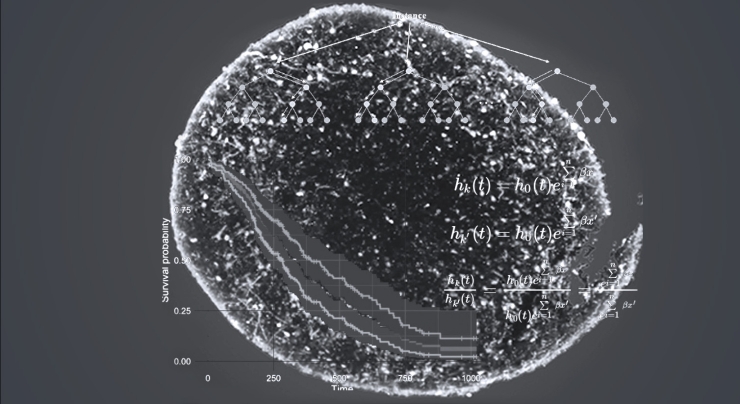
We then layer on advanced machine learning and bioinformatics techniques to quantify the features of disease in the model. Now we’re ready to discover new therapeutic targets that can be drugged to restore healthy neuronal function in these patient models.
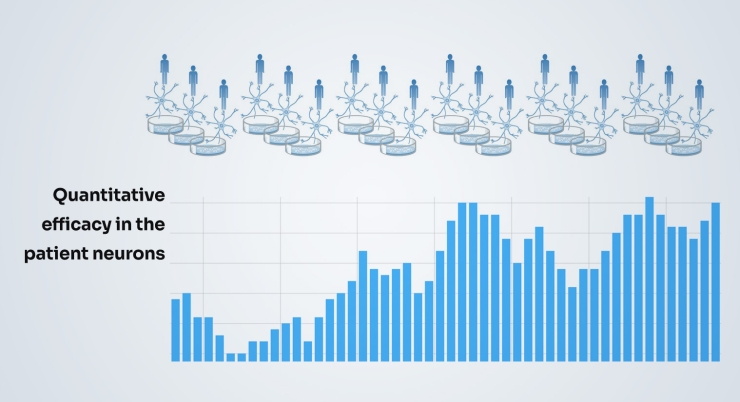
Next, we scale this up massively and test the target across neurons from many patients, essentially a “clinical-trial-in-a-dish” and see how well the new drug target works across different patient types including sporadic and genetically defined forms of disease.
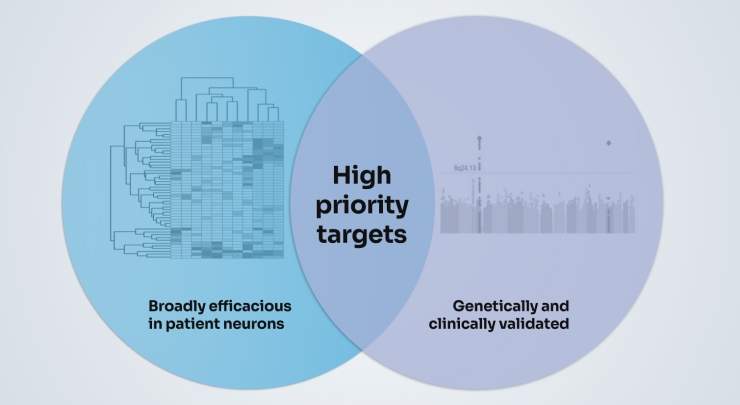
We also layer on evidence from large scale genetic studies, studies of post-mortem tissue and clinical evidence. Sometimes we start with evidence from our studies and validate with the clinical evidence, and sometimes we do the reverse.
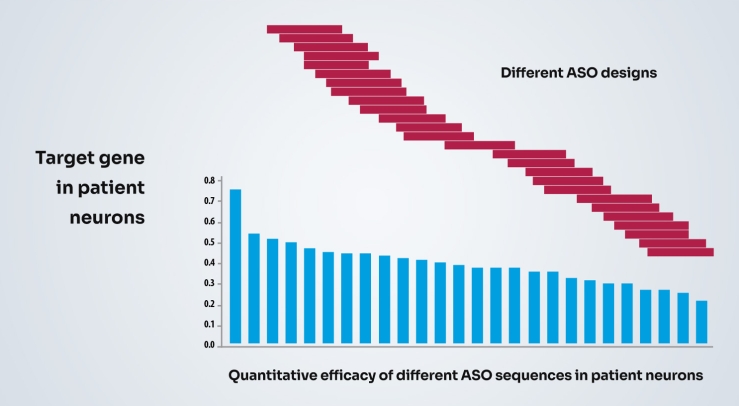
Once we have selected a therapeutic target, we can make new drugs and optimize the drugs using the patient models. Often small changes in the drug design can make big differences in efficacy in the patient models.
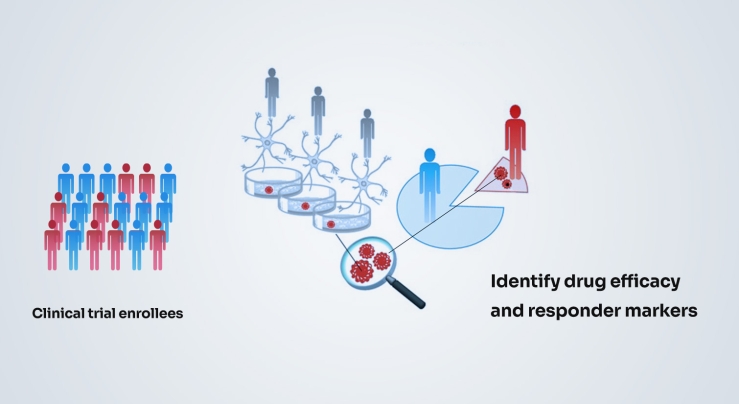
Mechanism of action studies in the patient models can help us develop target-engagement biomarkers to guide dosing in the clinic, and possibly also provide criteria for a more targeted trial that is more likely to succeed.