Therapeutic Pipeline
AcuraStem has utilized its patient-based approach and iNeuroRx® technology platform to advance highly promising therapeutic candidates for the treatment of ALS and FTD. AcuraStem is confident these treatments will be effective for diverse forms of disease, not limited to rare, genetically defined subsets (e.g. C9ORF72 - ALS / FTD).
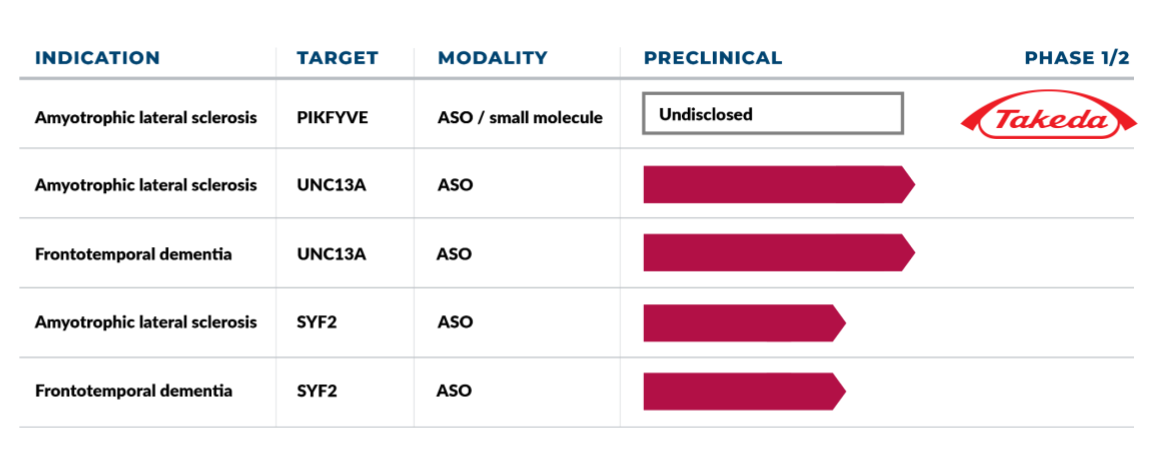
AcuraStem has rapidly established the growing pipeline of therapeutics shown below for advancement into human trials.
PIKFYVE: a novel therapeutic target for amyotrophic lateral sclerosis and frontotemporal dementia
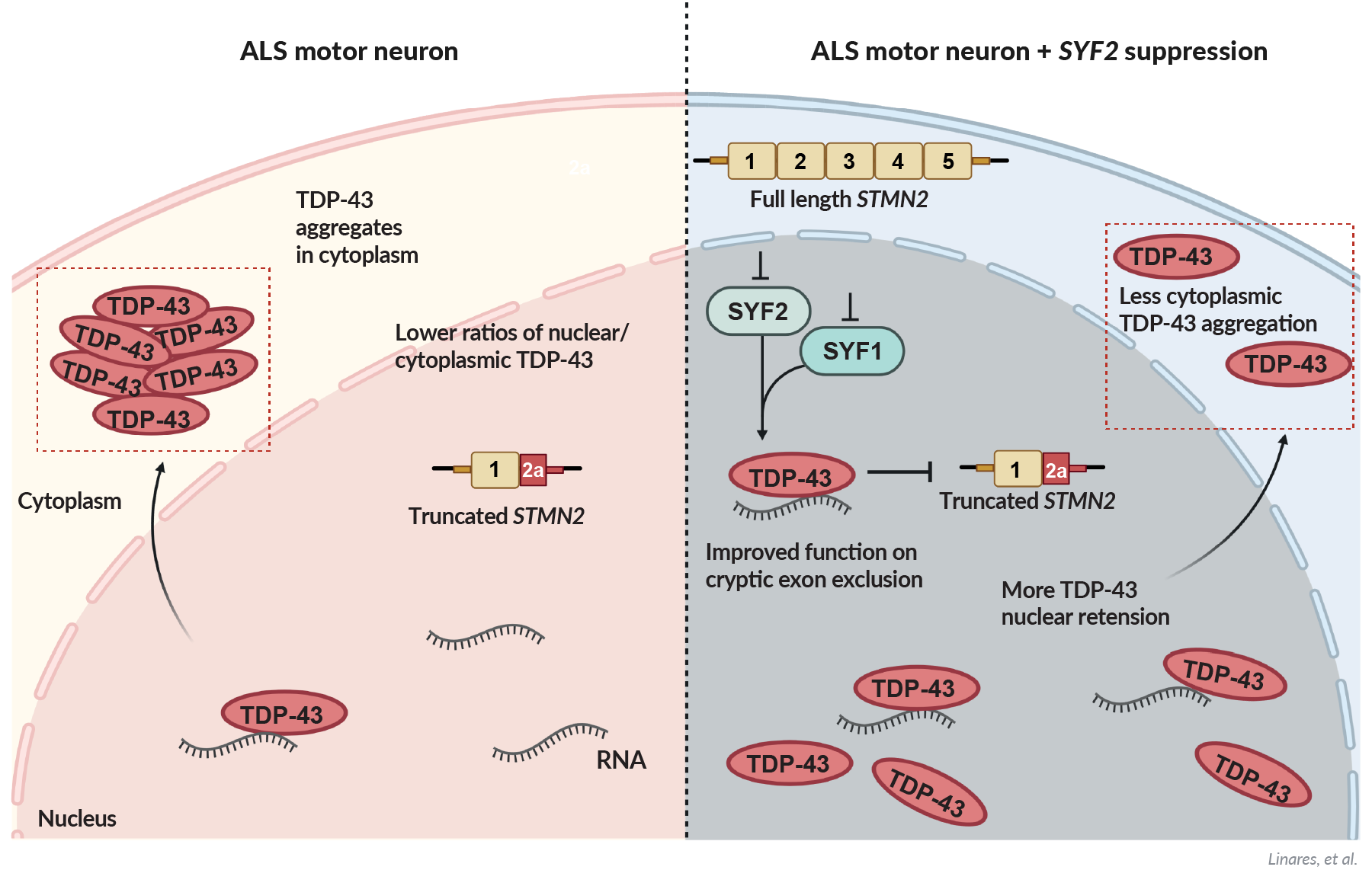
The therapeutic potential of PIKFYVE for treating ALS and FTD was discovered through a collaboration of our co-founders prior to the formation of AcuraStem. This research was led by Dr. Justin Ichida’s laboratory at USC and published in Nature Medicine in 2018. After many years of further collaboration between the labs of AcuraStem and Dr. Ichida, our data shows that suppression of PIKFYVE with an antisense oligonucleotide (ASO) therapeutic prevents neurodegeneration by activating multivesicular body exocytosis to secrete toxic protein aggregates and restore healthy neuronal function.
High levels of accumulated proteins such as phosphorylated TDP-43 and tau aggregates are toxic to neurons and our PIKFYVE ASO reverses that in many preclinical models of ALS and FTD
Researchers have long studied methods to enhance cellular autophagy (degradation of unwanted proteins), which declines with aging and involves biological pathways impacted by mutations known to cause neurodegeneration, but success has been elusive. Patient-based approaches, initially at Dr. Ichida’s lab and later at AcuraStem, have discovered PIKFYVE suppression and exosomal secretion as a novel way to augment autophagy and prevent neurodegeneration.
UNC13A: a highly genetically validated target for TDP-43 proteinopathies
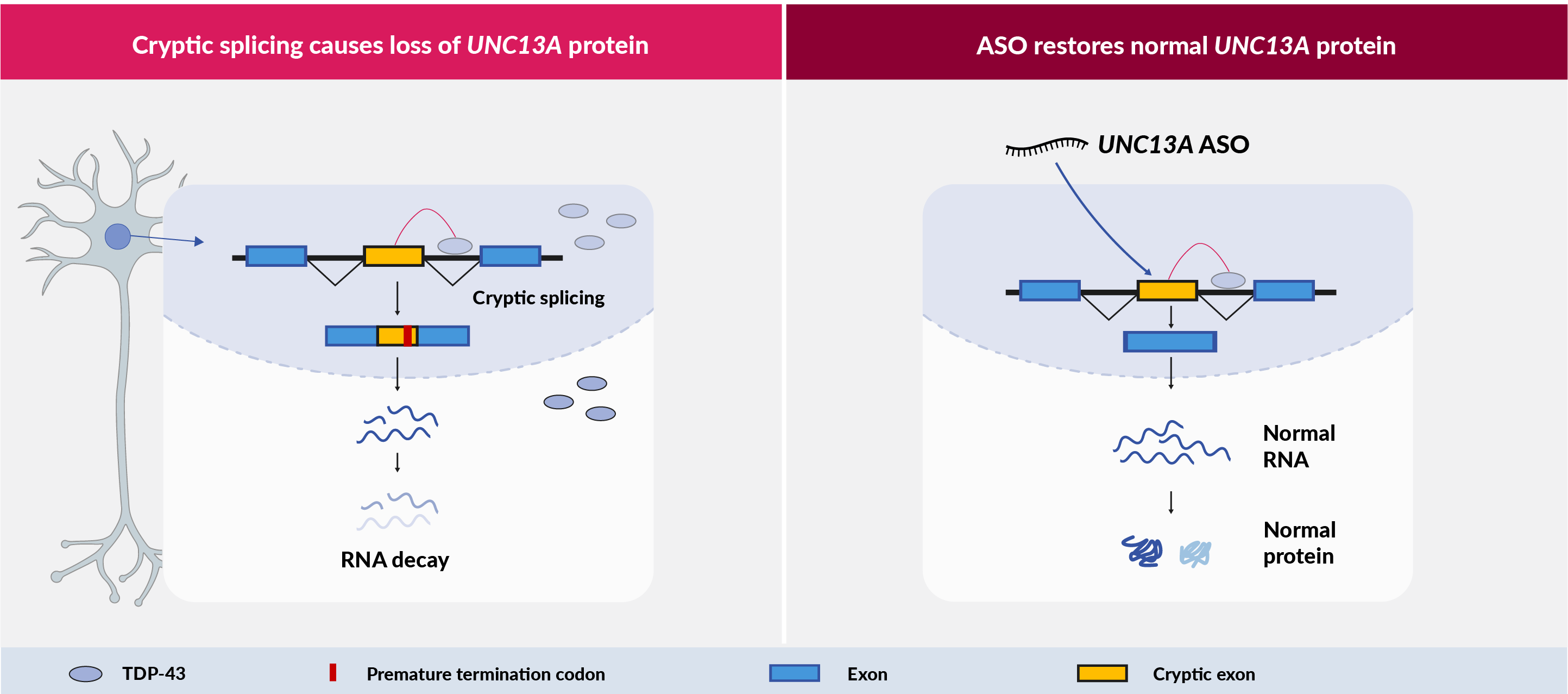
Around 97% of ALS cases, 45% of FTD cases and 57% of Alzheimer’s cases have TDP-43 pathology. TDP-43 is a RNA-binding protein normally located in the nucleus, but in patients it is depleted in the nucleus and forms into toxic aggregates in the cytoplasm of neurons. A variant in the UNC13A gene has been known for many years as one of the strongest risk factors for ALS and FTD, but its role in disease was unknown. Recent discoveries have shown that when TDP-43 is lost from the nucleus, a RNA processing error occurs that introduces a piece of intron, known as a cryptic exon, into the UNC13A messenger RNA (mRNA) leading to a loss of UNC13A protein. The UNC13A risk variant is located very close to the TDP-43 binding site and exacerbates this UNC13A pathology. Similarly the risk variant is predictive of reduced survival in ALS and FTD patients. This link between patient outcomes and UNC13A pathology strongly suggests that UNC13A dysregulation is central to disease progression in TDP-43 proteinopathies.
We discovered that patient neurons on our iNeuroRx® platform recapitulate the UNC13A pathology and we were able to quickly design and test ASOs that can potently suppress the cryptic exon and restore normal UNC13A function.
iNeuroRx® provides the ideal platform to develop ASO drug candidates with optimal efficacy, and also to learn how UNC13A pathology drives disease in ALS and FTD. This mechanistic understanding will be key for the development of biomarkers to guide dosing in patients.